In a Nature paper, CeMM researchers report on the epigenetic and transcriptional regulation in structural cells. They found widespread activity of immune genes, suggesting that structural cells are deeply involved in the body’s response to pathogens. Moreover, the study uncovered an epigenetic potential that pre-programs structural cells to engage in the immune response against pathogens. These findings highlight an underappreciated part of the immune system and open up an exciting area for research and future therapies.
The immune system protects our body from constant attack by viruses, bacteria, and other pathogens. Much of this protection is provided by hematopoietic immune cells, which are derived from the bone marrow and specialize in fighting pathogens. They include macrophages, which remove pathogens; T cells, which kill infected virus-producing cells; and B cells producing antibodies that neutralize pathogens. However, immune functions are not restricted to these ‘specialists,’ and many more cell types are able to sense when they are infected and contribute to the immune response against pathogens.
Structural cells provide essential building blocks of the body and play an important role in shaping the structure of tissue and organs. Most notably, epithelial cells constitute the surface of the skin, while also separating tissues and organs from each other; endothelial cells coat the inside of all blood vessels; and fibroblast provide the connective tissue that keeps tissues and organs in shape. Structural cells are often regarded as simple and rather uninteresting components of the body, despite their well-established roles in autoimmune diseases (such as rheumatoid arthritis and inflammatory bowel disease) and in cancer. In their new study, Thomas Krausgruber, Nikolaus Fortelny and colleagues in Christoph Bock’s laboratory at CeMM focused on elucidating the role of structural cells in immune regulation by pursuing a systematic, genome-wide analysis of epigenetic and transcriptional regulation of structural cells in the body.
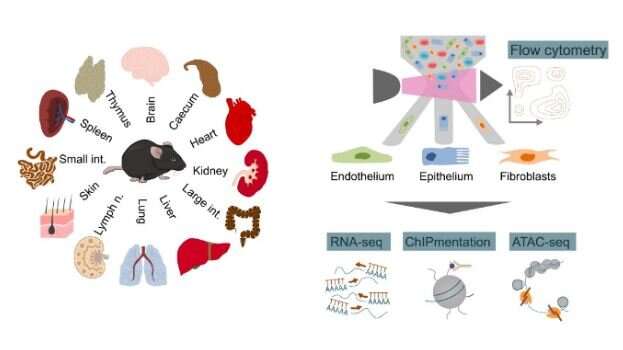
To that end, the CeMM researchers established a comprehensive catalog of immune gene activity in structural cells, applying high-throughput sequencing technology (RNA-seq, ATAC-seq, ChIPmentation) to three types of structural cells (epithelium, endothelium, fibroblasts) from 12 organs in healthy mice. This dataset uncovered widespread expression of immune genes in structural cells as well as highly cell-type-specific and organ-specific patterns of gene regulation. Bioinformatic analysis detected genes that control a complex network of interactions between structural cells and hematopoietic immune cells, indicating potential mechanisms by which structural cells contribute to the response to pathogens.
Interestingly, many immune genes showed epigenetic signatures that are normally associated with high gene expression, while the observed expression in structural cells obtained from healthy mice was lower than expected based on their epigenetic signatures. CeMM researchers therefore hypothesized that these genes are epigenetically pre-programmed for rapid upregulation when their activity is needed—for example in response to a pathogen. To test this hypothesis, they joined forces with Andreas Bergthaler’s laboratory at CeMM, capitalizing on their expertise in viral immunology and infection biology.
When the mice were infected with a virus (LCMV) that triggers a broad immune response, many of those genes that were epigenetically poised for activation became upregulated and contributed to the transcriptional changes that structural cells showed in response to viral infection. These results suggest that structural cells implement an “epigenetic potential” that pre-programs them to engage in rapid immune responses. As an additional validation, the researchers triggered an artificial immune response by injecting cytokines into mice, and they indeed found that many of the same genes were upregulated.
Source: Read Full Article
