A researcher team from Ecole Polytechnique Federal de Lausanne led by Dr. Vivek Thacker now group leader at the Department of Infectious Diseases at Heidelberg University Hospital have studied why tuberculosis bacteria form long strands and how this affects their infectivity. Their findings could lead to new therapies and have been published in the journal Cell.
Mycobacterium tuberculosis, the causative agent of tuberculosis, causes an estimated 1.6 million deaths worldwide each year. The outcome of infection ranges from clearance without any disease to chronic infection with emaciation and death. This wide variability is largely dependent on the immune response of the infected host.
The analysis of the pathogen-host interplay is a central research topic of the Department of Medical Microbiology and Hygiene at the Department of Infectious Diseases at Heidelberg University Hospital (UKHD).
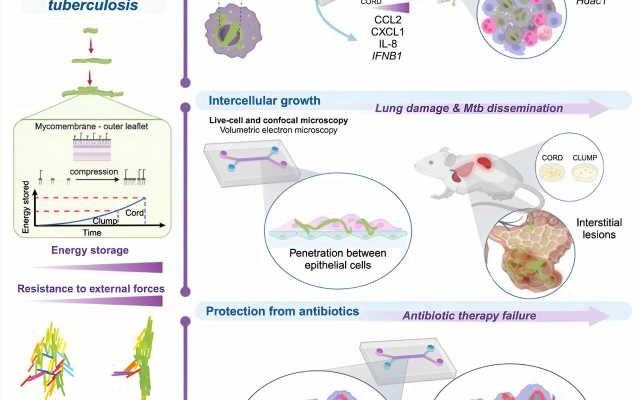
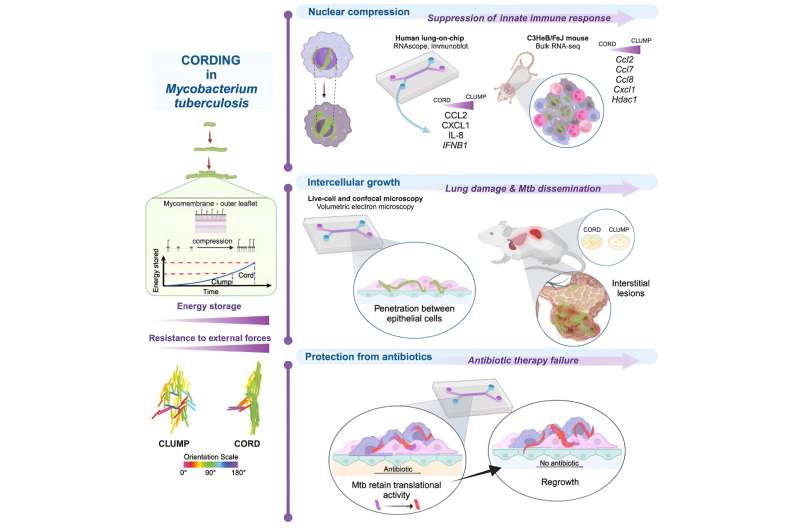
It has long been known that Mycobacterium tuberculosis produces cord factor, which is important for the pathogen’s disease-causing properties, its virulence, and which also leads to a special growth behavior: Mycobacterium tuberculosis can produce densely packed strands of many bacteria that can be observed by light microscopy. How these strands or cords contribute to the pathogenic properties of Mycobacterium tuberculosis was essentially not understood until now
In the current work, scientists led by Dr. Vivek Thacker, have for the first time established a link between this “mechanical” growth behavior and the infection biology of Mycobacterium tuberculosis. In an interdisciplinary research approach, they used live cell microscopy and synthetic organ models (“lung-on-chip”).
Bacterial strands constrict cell nucleus
“We were able to show that bacterial compression leads to the storage of energy via cord factor in the pathogen’s membrane,” Thacker reports. “This allows pathogens that have been taken up by phagocytes in the lungs and that should be destroyed to grow in strands even within these defense cells.”
Dr. Vivek Thacker’s team observed that this growth constricts and strangulates the nucleus of the host cell. As a result, the cell activates defense mechanisms to a lesser extent and therefore cannot contain the pathogen. Furthermore, the rigid architecture of cords outside cells leads to the pathogen being able to spread more easily between the epithelial cells lining the alveoli and to other organs in the body
Finally, the research team observed that bacteria in such cords are protected from the effects of antibiotics and can grow again after antibiotic therapy has ended.
New research field ‘mechanopathology’
“We have succeeded in linking mechanical growth behavior at the cellular level with biological functions,” says Dr. Vivek Thacker. “Mechanopathology is a promising new field of research.”
Some aspects of this mechanical growth behavior are reminiscent of biofilms formed by other important infectious disease pathogens, such as staphylococci and pseudomonads, which similarly contribute to insensitivity to antibiotics and inhibition of host defenses. Linking physical growth behavior and infection biology is thus a highly interesting area of research that could provide new insights for the development of much-needed therapies.
“I am pleased that with Dr. Vivek Thacker we have been able to attract an excellent scientist to work here in Heidelberg” says Professor Dr. Alexander Dalpke, Medical Director of the Department of Medical Microbiology and Hygiene.
“Dr. Vivek Thacker ideally complements the research focus of the Institute and the Center for Infectious Diseases. In particular, in the spirit of Ruperto Carola, he builds bridges to neighboring research areas, such as the new Faculty of Engineering. His sophisticated work with live cell microscopy and synthetic organ models is forward-looking and strengthens infectious medicine in Heidelberg.”
More information:
Richa Mishra et al, Mechanopathology of biofilm-like Mycobacterium tuberculosis cords, Cell (2023). DOI: 10.1016/j.cell.2023.09.016
Journal information:
Cell
Source: Read Full Article