The Native Antigen Company, part of LGC Clinical Diagnostics and one of the world’s leading suppliers of reagents that enables research into diagnostics and vaccines for emerging and endemic infectious diseases, today announced the launch of its SARS-CoV-2 Neutralisation Assay Development Kit. The kit can be used to identify and qualitatively assess the ability of antibodies to neutralize SARS-CoV-2-receptor binding, to support research into variants and their effects on natural and vaccine-induced patient immunity.
SARS-CoV-2 Assay. Image Credit: The Native Antigen Company
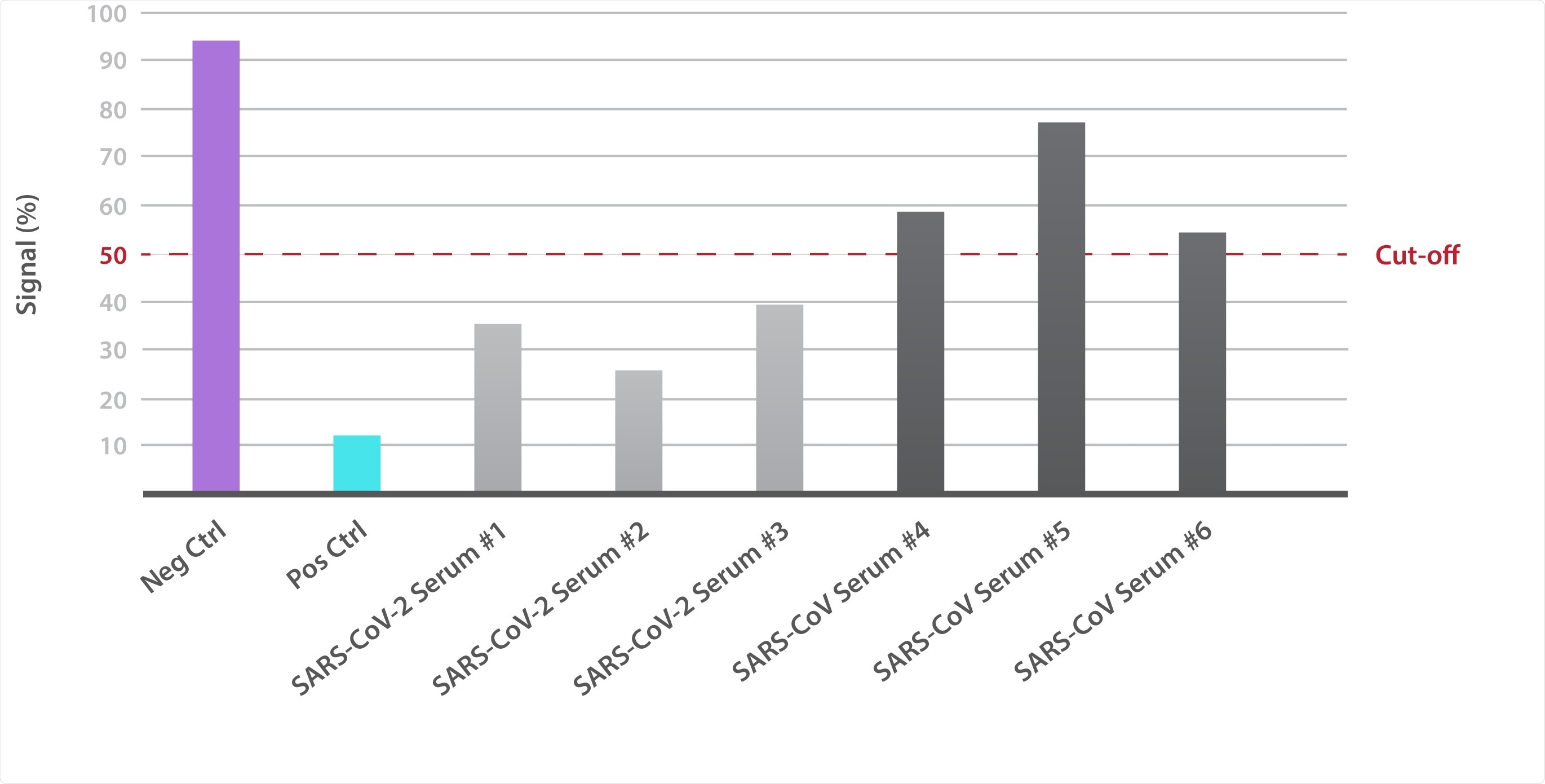
The easy-to-use kit contains all the key reagents required to measure SARS-CoV-2-antibody binding, including the Spike receptor-binding domain (RBD) of the prototypic Wuhan-Hu-1 strain, labeled ACE2, and positive and negative monoclonal antibody controls. These reagents enable the assessment of neutralizing activity of patient and therapeutic antibodies and can be used alongside The Native Antigen Company’s growing range of SARS-CoV-2 variant Spike proteins to assess differences in antibody/ACE2 affinities and competitive binding.
This marks The Native Antigen Company’s first release of a dedicated kit for the development of neutralization assays. Our data demonstrates the SARS-CoV-2 Neutralisation Assay Development Kit’s effectiveness and we are confident in its ability to support vital research and development efforts for public health.”
Dr. Andy Lane, Commercial Director, The Native Antigen Company
The Native Antigen Company’s in-house data shows that the COVID-19 patient sera is able to effectively neutralize the RBD and prevent it from binding to the human ACE2 cell surface receptor.
For further information about The Native Antigen Company’s SARS-CoV-2 Neutralisation Assay Development Kit, please visit: https://thenativeantigencompany.com/sars-cov-2-neutralisation-assay-development-kit-now-available/
