Northwestern Medicine scientists have discovered a potential therapeutic target in the donor lung that can prevent primary graft dysfunction (PGD) in lung transplant recipients, according to findings published in the Journal of Clinical Investigation (JCI).
GR Scott Budinger, MD, chief of Pulmonary and Critical Care in the Department of Medicine and the Ernest S. Bazley Professor of Airway Diseases, was senior author of the study.
“Among solid organ transplants, lung transplant success rates are low, with only half of patients surviving beyond five years—and an important driver of early and later complications after lung transplant is PGD,” said Budinger, who is also a professor of Cell and Developmental Biology.
Northwestern scientists have been working to address PGD, a potentially lethal injury to the fragile transplanted lung that happens in the first days after the operation, for years.
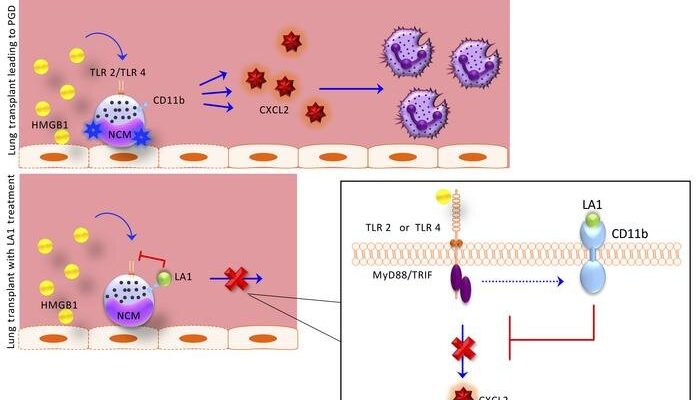
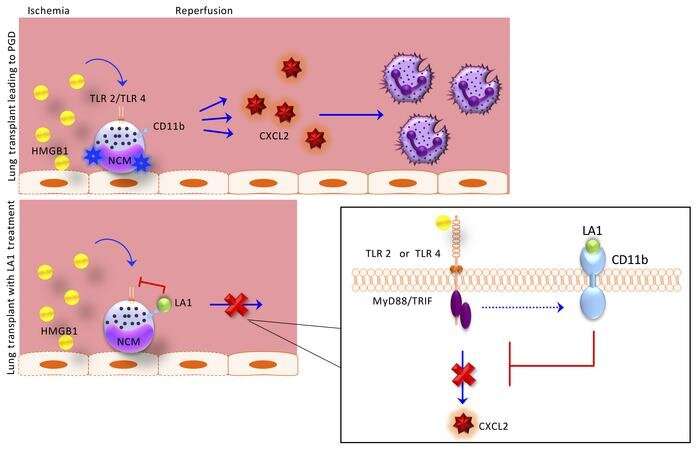
In 2017, they published a critical discovery: After transplant, blood immune cells called non-classical monocytes (NCMs) retained in the donor lung activate a pathway that attracts damaging neutrophils from the host into the newly transplanted lung. Those findings suggested that targeting NCMs in donor lungs could potentially prevent the development of PGD. Yet, the treatments options that grew out of that research remained limited.
“Most of the treatment options available for PGD are symptomatic treatments, which do not necessarily treat the actual disease process itself. So, it was critical to elucidate the mechanism further in order to even consider disease-modifying therapeutics,” said the study’s first author, Melissa Querrey, Ph.D., a fourth-year student in the Medical Scientist Training Program (MSTP).
With the current study, the team has taken the initial research (led by Ankit Bharat, MBBS, chief of Thoracic Surgery in the Department of Surgery and the Harold L. and Margaret N. Method Professor of Surgery, who is also a co-author of the JCI study) a step further, finding the pathways that activate these cells after transplant. Even more importantly, they found that a protein on these cells, CD11b, can act as a molecular “brake” to reduce their activation.
A critical finding of this paper is that an activator of the CD11b braking system LA-1, administered only to the donor lung before transplant, markedly reduced the severity of transplant induced early lung injury, according to Budinger.
“This is important because treatment of the donor lung before transplant is possible using ex vivo perfusion or ‘lung in a box’ systems,” said Budinger. “Since the recipient never needs to receive the drug, this strategy is likely to reduce any side effects of the drug.”
In addition to moving this therapeutic approach forward in the ex-vivo perfusion system, investigators have their sights set on potential larger-scale benefits.
Source: Read Full Article