A study of lung tissue from patients with end-stage bronchiolitis obliterans syndrome (BOS) as a complication of lung transplantation yielded molecular and morphologic insights into the development of chronic rejection. These insights may help develop therapies to optimize long-term outcomes for lung transplantation patients, report investigators in The American Journal of Pathology.
BOS, a common form of chronic lung allograft dysfunction, limits long-term survival after lung transplantation. This study improves our understanding of the formation of obliterative bronchiolitis (OB) lesions in small airways. Identification of early processes leading to BOS and improved understanding of the underlying mechanisms will help us develop tools to manage BOS.
“Lung transplantation can be the last option for some end-stage patients with various underlying lung diseases. Therefore, improving transplantation outcomes may have a significant impact on their survival,” explained lead investigator Gunilla Westergren-Thorsson, Ph.D., Lung Biology Unit, Department of Experimental Medical Science, Lund University, Lund, Sweden. “Identifying the underlying initial cause of OB lesions in patients undergoing lung transplantation is key to developing therapies to reduce the chance of chronic allograft dysfunction.”
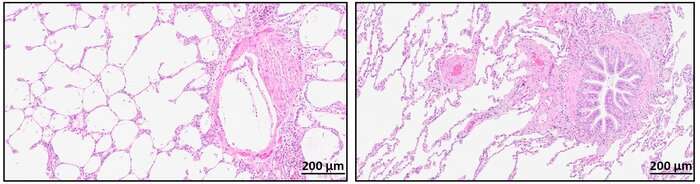
Investigators examined the protein composition in lung tissue from four patients with end-stage BOS as a complication of lung transplantation using laser-capture microdissection and optimized sample preparation protocols for mass spectrometry. Immunohistochemistry and immunofluorescence were used to determine the spatial distribution of commonly identified proteins at the tissue level. Protein signatures were established for 14 OB lesions, which showed variations in their protein content as well as exhibited common features.
The study concluded that the OB lesions were heterogeneous and differed within and among patients. The protein patterns in the lesions were correlated to pathways of extracellular matrix organization, tissue development, and wound healing processes. These results encourage further research into ways to affect modulating these cellular pathways and events.
“This study shows a link between the morphological appearance of OB lesions in remodeled airways in lungs from patients suffering from end-stage BOS and their respective protein content, providing molecular and morphological insights into the development of chronic rejection after lung transplantation,” noted Dr. Westergren-Thorsson.
Although a multitude of proteins (up to 89 proteins per lesion) were identified by mass spectrometry, basement membrane proteins were overrepresented and accumulated outside the usual epithelial basement membrane. Since basement membranes provide cell and tissue support and act as a platform for complex cell signaling, these results suggest an epithelial/mesenchymal imbalance in the OB lesion.
“The aberrant accumulation of extracellular matrix proteins results in an increased stiffness of the microenvironment in OB lesions that creates a vicious circle in which mesenchymal cells respond to the reduced compliance and increase in rigidity by producing an excess amount of extracellular matrix. These structural changes contribute to the lesions and further propagate the obliterations,” Dr. Westergren-Thorsson said.
These findings are particularly important because survivors of COVID-19 who have developed chronic lung fibrosis and have a poor prognosis may need lung transplantation.
“The global impact of the pandemic will propagate over several years to come with long-term lung fibrosis following the initial COVID-19 infection. Our study encourages research into ways to affect these cellular pathways and events to optimize the long-term outcome for lung transplantation,” Dr. Westergren-Thorsson concluded.
Source: Read Full Article
