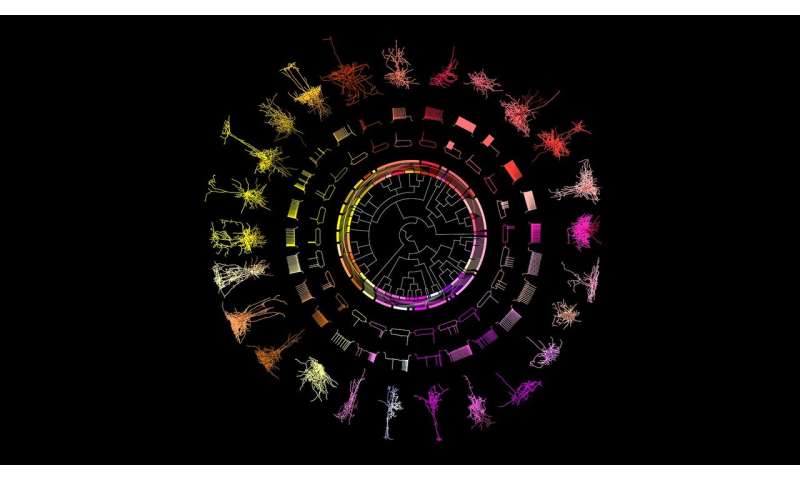
A new lens on visual neurons is laying the groundwork for a more complete “family tree” of the mammalian brain. A team of researchers from the Allen Institute for Brain Science, a division of the Allen Institute, published a study—the largest of its kind to date—in the journal Cell today revealing a new categorization of mouse neurons that relies on multiple types of data drawn from each individual cell.
Just as biologists sketch evolutionary trees whose branches represent the development of different species and the relationships between them, some scientists now look to cells to draw another kind of family tree. This tree describes not the millions of species that inhabit our planet, but the millions of neurons that make up the kumquat-sized brain of the lab mouse.
Before the advent of DNA sequencing, scientists used visual cues to define species. Does the animal have fur or feathers? A beak, talons, hooves? The same is true for early studies of the brain—over the past 100 years, neuroscientists have realized that our neurons and other brain cells come in a wide variety of shapes and sizes. But detailed visual descriptions of these cells have failed to reveal many insights about how different neurons carry out different jobs, let alone how individual cells might change in disease.
The Allen Institute team used a special technique to simultaneously capture a brain cell’s 3-D shape, its unique electrical properties, and the suite of genes it switches on, from hundreds of individual neurons from the part of the mouse brain that processes visual information. Because this technique captures multiple types of data from each single cell, the researchers can use this dataset to classify the neurons into different types, part of their larger effort to build a “periodic table” of the mammalian brain. The data and the cell-classification scheme can link together other studies that focus on just one characteristic, resulting in a more holistic understanding of different brain cells and their relationships.
By classifying brain cells into categories, or cell types, and studying how and where those types of cells do their jobs, researchers hope to understand more about how the brain works as a whole—and where it might go wrong in disease.
“It’s becoming increasingly clear that in some disorders, there’s a deficiency in a very particular type of neuron in a particular part of the brain,” said Gabe Murphy, Ph.D., Associate Director of Electrophysiology at the Allen Institute for Brain Science. “The more we understand the different types of neurons that exist and what makes them unique, the more we can understand what goes wrong if you have vulnerabilities in one or more of those types in disease.”
Murphy led the study along with the Allen Institute for Brain Science’s Hongkui Zeng, Ph.D., Executive Vice President and Director; Nathan Gouwens, Ph.D., Assistant Investigator; and Staci Sorensen, Ph.D., Associate Director of Neuroanatomy.
“This study has essentially provided a ‘lookup table’ for other neuroscientists, such that if you have information about only one property of a neuron, you can infer the other properties,” said Edward Callaway, Ph.D., Professor at the Salk Institute for Biological Studies and an advisor to the Allen Institute for Brain Science, who was not involved in the study. “We need this kind of linking study to figure out what are the true cell types of the brain so we can begin to characterize them. This dataset enables that linking, and it will no doubt prove a great resource for future discovery.”
The Allen Institute researchers focused on inhibitory neurons, the type of cells that put the brakes on other neurons’ activity. Their study binned these neurons into 28 different types. Scientists had not previously realized this level of diversity existed in visual inhibitory neurons.
Revealing new patterns in the brainNeuroscientists use characteristics like a neuron’s shape, the number and type of connections it makes with other neurons, the kinds of electrical signals it sends and receives, and the genes it switches on, to sort the cells into cell types. Most studies focus on just one of these attributes. The Allen Institute researchers used a technique known as Patch-seq to capture three from a single cell: a neuron’s 3-D shape, or morphology; its unique electrical properties, or electrophysiology, which researchers record by “patching” onto the cell with a glass electrode; and the sequences of genes it switches on, or its transcriptomic profile.
Patch-seq is a complex technique. To generate this large dataset, the Allen Institute team set up a “pipeline” to ensure their results were standardized and high-quality. The team captured electrical and gene expression data from more than 4,200 neurons in the current study. Because building full 3-D morphologies is time-intensive, they focused on 517 neurons to trace. This step was carried out with input from gamers playing the free neuroscience game Mozak, which was developed by researchers at the Allen Institute and the University of Washington Center for Game Science.
It’s a 21st century twist on a branch of neuroscience that traces back to 19th-century scientists who first described many neurons by their shape.
“With this approach, we’re now learning something new about the brain by adding transcriptomics to the method of studying neuron morphology that’s been around for more than 100 years,” Sorensen said. “Using transcriptomics to label cell types has been revolutionary. When we sort cells in this way, we’re able to reveal patterns we hadn’t noticed before.”
Source: Read Full Article
