A century ago, German pathologist Otto Aichel discovered that tumor cells could fuse with immune cells, forming fusion cells with both immune cell motility and tumorigenic ability of tumor cells, which are more likely to spread and metastasize through the circulatory system. Due to the limitation of currently available approaches, such fusion cells are difficult to be isolated and analyzed thoroughly.
A new study published in Lab on a Chip on June 28 proposed a novel hydrodynamic structure to achieve one-step and label-free isolation of circulating tumor cells (CTCs) and fusion cells (CFCs) from whole blood. The integrated microfluidic platform isolated rare CTCs and CFCs with high purity and high cell viability, enabling direct downstream analysis with single-cell RNA sequencing. The study was led by Dr. CHEN Yan’s group from Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences.
In this study, researchers proposed a filter deterministic lateral displacement (filter-DLD) concept to achieve one-step and label-free CTCs and CFCs isolation. The novel hydrodynamic structure is designed and simulated by multiphysics finite element analysis, which enables precise manipulation of cell motion.
The filter-DLD structure not only has a lower critical cell separation size than conventional DLD designs, but also achieves a higher depletion rate of smaller red blood cells, which make up the largest proportion of blood.
By combining the filter-DLD concept and the cascaded chip design, researchers fully explored the tremendous potential of rare cell sorting based on physical properties. The integrated microfluidic platform demonstrated excellent performance for size-based cell separation, and achieved high separation efficiency (>96%), high cell purity (WBC removal rate 99.995%), high cell viability (>98%) and high processing rate (1mL/min).
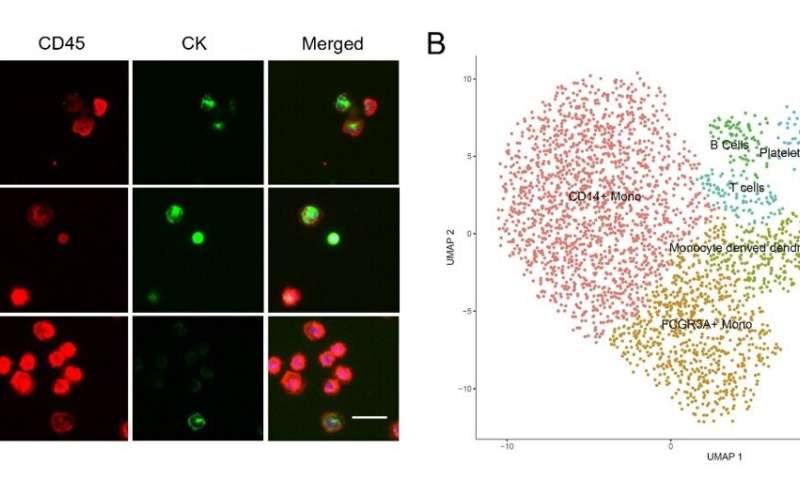
Using this platform, researchers analyzed samples from non-small cell lung cancer patients. CTCs and tumor cell-leukocyte fusion cells (CFCs) were efficiently collected, and changes in CTCs levels were used for treatment response monitoring. Due to the extremely high viability of the enriched cells, downstream analysis can be performed directly with high-throughput single-cell RNA sequencing to study cancer driver genes and heterogeneity. The results showed that there were more CFCs than CTCs in the peripheral blood of cancer patients. CFCs are expected to reveal new mechanisms of tumor evolution and metastasis, and provide a novel marker for early diagnosis, prognosis and monitoring of tumors.
Source: Read Full Article
