If vision science were a movie, the iris would be a supporting actor. It doesn’t get as much limelight as the retina, the eye’s light-sensing tissue. And it’s not as high-profile as the lens, which can cloud with cataracts as people age.
Though the iris—the colorful tissue that rings the pupil—comes in a rainbow of showy shades, for most scientists, “it was not the main attraction in the eye,” says Jeremy Nathans, a Howard Hughes Medical Institute Investigator at Johns Hopkins University School of Medicine. In fact, he has spent most of his career studying the molecular biology of the retina.
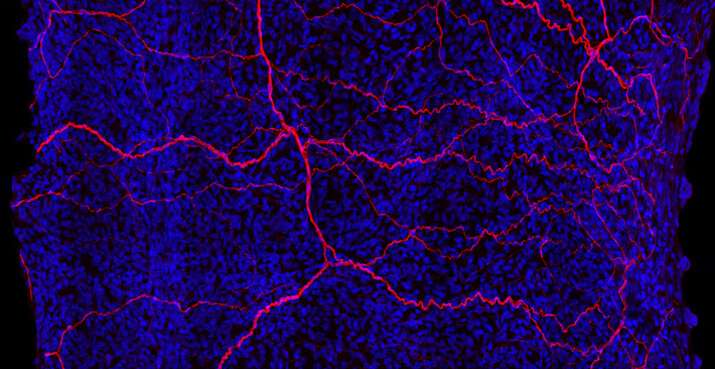
“The basic biology of the iris had kind of languished,” Nathans says. Not anymore. He and his colleagues Jie Wang and Amir Rattner have now developed a high-resolution map of the mouse iris that distinguishes individual cells by the activity of their genes. The trio was motivated by the beauty of the iris, the diversity of iris structure in different animals, and its importance for vision, Nathans says.
His team identified eight types of iris cells and uncovered new information about iris cell development and the genes that switch on when the pupil dilates, they report November 16, 2021, in the journal eLife. The researchers’ cell-by-cell map of the iris could one day help identify genes involved in iris-related eye disorders. The work could also guide engineering of healthy iris cells used to replace diseased cells elsewhere in the eye.

“This is the first comprehensive and in-depth study of mouse iris tissue,” says Bo Chen, an eye researcher at the Icahn School of Medicine at Mount Sinai who wasn’t involved in the research. “It’s an important foundational paper.”
The iris is a thin sheet of tissue, roughly blueberry-sized in humans, that appears in shades from icy blue to hues of deepest brown. A hole at the iris’s center, the pupil, expands and contracts to control the amount of light entering the eye.
In mice, the iris contains about 30,000 cells. Nathans and his team examined thousands of these cells, looking inside nuclei to catalog which genes were turned on. This let the team identify different cell types based on their active genetic material and the location of the cells in the iris.
The mapping effort presented Nathans and his team with a surprise: the iris sphincter muscles that form a ring around the pupil come in two cell types, as do the stroma cells that act as fillers between other cells. Until now, scientists didn’t know that these cellular subtypes existed, Nathans says.
Next, the researchers wondered if iris cells’ genetic activity might change when the pupil expanded or constricted. The team compared cells of a relaxed iris with those experimentally dilated or constricted using eye drops.
When the pupil widened to let in more light, iris cells squished together, like the folds of a zig-zag window shade pulled all the way up, Nathans says. This compression switched on a new set of genes—a response that may be triggered by mechanical stress to the cells, he says.
Those changes occurred mainly in iris muscle cells that dilate the pupil, and they happened fast—within 30 minutes of administering the eye drops. Chen says that the rapid change in gene activity in a healthy iris is a novel finding. Usually, he says, “I only think of this happening in response to an injury or disease.” Nathans and his colleagues suspect that the gene activity changes help the iris transition from a relaxed to a dilated state.
The team’s research also demystifies the origin of iris cells, a controversial topic in the vision science field. “You might think a bunch of cells in the embryo organize themselves in the right place and become the eyeball,” Nathans says. “But it’s not that simple.” The researchers traced the developmental lineage of iris cells and discovered that many originate from the neural crest. This transient group of cells migrates to various parts of the body and generates cells in different organs.
Source: Read Full Article
