Like differently shaped individual beads in a chain, amino acids are joined together to form proteins. The main function of amino acids is to serve as building blocks for proteins. Proteins tend to be typically comprised of between 50 to 2000 amino acids joined end-to-end in many different combinations.
Each protein has unique sequences of amino acids in its own twisted and folded configuration. The functions of proteins are vast and many, because they are virtually required for all cellular processes of normal physiological functioning.
There are 20 different amino acids that combine to create the impressive array of chemical versatility of proteins. Amino acids can be essential, nonessential or conditional. They are considered essential when required to be taken in by diet, whereas nonessential when they can be made by the body. Conditional amino acids are mostly required only during certain circumstances, such as stress and illness.
The manner or sequence in which these amino acids combine to form a protein determines the 3-dimensional structure and function, which is unique to the particular protein. Some of the functions of proteins include their roles as antibodies, enzymes, messengers and in transport/storage and structural capacities.
Antibodies
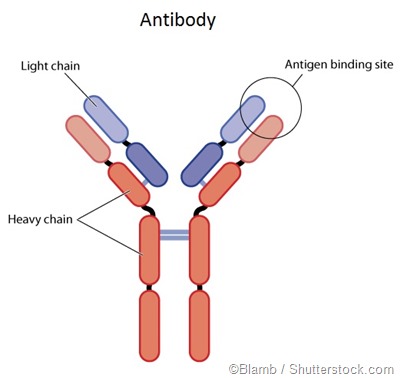
Antibodies are proteins that are produced by the immune system. They play a pivotal role in detecting antigens, which are complex proteins recognized by the body as foreign and harmful. Viruses, bacteria, fungi and parasites as well as dangerous chemicals are all examples of antigens.
In some unfortunate instances, antibodies may also be produced against healthy tissues when the body erroneously recognizes them as foreign. This phenomenon is known as an autoimmune disorder. Antibodies are unique and made with a high degree of specificity to defend against each different antigen that the body encounters.
Enzymes
Proteins that function as biological catalysts are called enzymes. They are primarily responsible for catalyzing or accelerating chemical reactions within the body by acting on molecules called substrates to produce products. Reaction rates are sped up by the lowering of the activation energy i.e., the minimum amount of energy required to initiate a reaction.
Unlike most other catalysts, enzymes are highly specific macromolecules. Their activity can be enhanced by molecules called activators and reduced by molecules known as inhibitors. Moreover, optimal conditions in temperature and pH are required if an enzyme is to function properly. Enzymes are found in every single organ and cell in our bodies – this includes, most notably, the blood and gastrointestinal tract.
Other functions
Proteins can exhibit a number of chemical messaging patterns in the form of hormones, neurotransmitters and neuropeptides. Hormones are produced by glands where they are subsequently transported by the circulatory system to regulate the behavior and physiology of distant organs and systems.
They are considered long range messengers. Unlike hormones, neurotransmitters are short range messengers that allow communication between a nerve cell and another target nerve, glandular or muscle cell. Neuropeptides are also short range messengers between nerve cells, however, unlike other neuronal messengers, neuropeptides are not recycled back into the cell once secreted.
Proteins constitute a fundamental part of cellular structure and support. Examples of structural proteins include collagen, keratin and elastin. Collagen is the fundamental component of connective tissue and is the most abundant protein in our bodies.
Alpha-keratin is vital in the formation of hair and nails, whereas elastin is a very elastic protein that enables tissues to regain their shape after some degree of deformation (e.g. contraction or stretch). On a greater scale, proteins found in muscles allow our bodies to move.
In addition to all of their aforementioned functions, proteins are capable of binding and carrying atoms as well as small molecules within cells and throughout our bodies. In this capacity they function as a form of storage and transport. Hemoglobin with the assistance of iron is one example of a protein transporter used to carry oxygen. An example of an intracellular storage protein is ferritin, which is needed to store iron.
Sources
- https://ghr.nlm.nih.gov/primer/howgeneswork/protein
- https://publications.nigms.nih.gov/structlife/chapter1.html#a1
- http://www.biology.arizona.edu/biochemistry/problem_sets/aa/aa.html
- www.khanacademy.org/…/chemistry-of-amino-acids-and-protein-structure
Last Updated: Feb 26, 2019

Written by
Dr. Damien Jonas Wilson
Dr. Damien Jonas Wilson is a medical doctor from St. Martin in the Carribean. He was awarded his Medical Degree (MD) from the University of Zagreb Teaching Hospital. His training in general medicine and surgery compliments his degree in biomolecular engineering (BASc.Eng.) from Utrecht, the Netherlands. During this degree, he completed a dissertation in the field of oncology at the Harvard Medical School/ Massachusetts General Hospital. Dr. Wilson currently works in the UK as a medical practitioner.
Source: Read Full Article
