The US Food and Drug Administration (FDA) has agreed to review a new drug application (NDA) for roflumilast foam 0.3% for treating seborrheic dermatitis in patients ages 9 years and older.
If approved, the once-daily, nonsteroidal foam would be the first topical drug with a new mechanism of action for seborrheic dermatitis in more than two decades, according to a press release from the manufacturer.
The target date for the FDA decision on approval — the Prescription Drug User Fee Act (PDUFA) date — is December 16, 2023.
The manufacturer, Arcutis Biotherapeutics, is developing topical cream and foam formulations of roflumilast, a highly potent and selective phosphodiesterase type 4 (PDE4) inhibitor for use in treating inflammatory dermatoses, particularly in areas of the body with hair such as the scalp, face, and trunk. The foam formulation of roflumilast is being developed for both seborrheic dermatitis and scalp and body psoriasis.
In July 2022, a 0.3% cream formulation of roflumilast (Zoryve) was approved for topical treatment of plaque psoriasis, in patients aged 12 years and older.
PDE4 is an enzyme that increases production of pro-inflammatory mediators and decreases production of anti-inflammatory mediators.
Decision Based on Phase 2, 3 Trials
The FDA’s decision to review the NDA is based on positive efficacy and safety data from the phase 2 and pivotal phase 3 trials of the foam, according to the company’s press release.
The STudy of Roflumilast foam Applied Topically for the redUction of seborrheic derMatitis (STRATUM) was the pivotal phase 3, parallel group, double-blind, vehicle-controlled study evaluating the safety and efficacy of the foam.
The trial, with 457 patients ages 9 and older with moderate to severe seborrheic dermatitis, met its primary endpoint with an Investigator Global Assessment (IGA) success rate of 79.5% in patients treated with the foam compared with 58% in those treated with vehicle at week 8 (P < .0001).
Improvement with roflumilast foam was seen early. At week 2, it demonstrated a statistically significant improvement compared with vehicle on IGA success, the company said. At week 8, 51.3% of patients in the roflumilast foam arm reached complete clearance, the company reports.
The foam was well-tolerated, according to the company. The incidence of treatment-emergent adverse events (TEAEs) was low and similar between active treatment and vehicle, and TEAEs were assessed as mild to moderate.
In the phase 2 and phase 3 studies, more than 90% of patients randomly assigned to treatment with roflumilast foam completed the 8 weeks of treatment, and the rates of discontinuations related to adverse events were low, 0.9% and 2.2% in the roflumilast foam vs vehicle groups, respectively, according to the company.
The most common side effects, affecting 1% or more of treated patients, were nasopharyngitis (1.5%), nausea (1.3%), and headache (1.1%).
The foam also demonstrated statistically significant improvement over vehicle on all secondary endpoints, including itch, scaling, and erythema, according to the company. More than 60% of people in the roflumilast foam group achieved an itch response at week 8 (62.8% with roflumilast foam vs 40.6% with vehicle; P = .0001), and significant improvements in itch were reported at weeks 2 and 4.
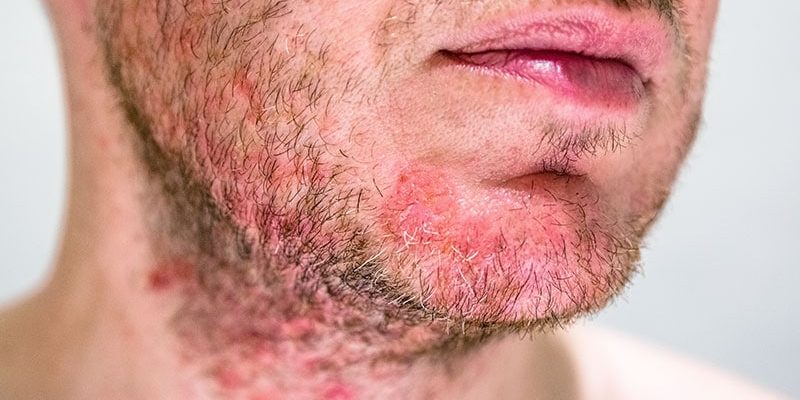
Seborrheic dermatitis is a common, chronic, and recurrent inflammatory skin disease that causes red patches covered with large, greasy, flaking scales, and persistent itch. It is found most often in areas of the body with oil-producing (sebaceous) glands, including the scalp, face, upper chest, and back.
Marcia Frellick is a freelance journalist based in Chicago. She has previously written for the Chicago Tribune, Science News, and Nurse.com, and was an editor at the Chicago Sun-Times, the Cincinnati Enquirer, and the St. Cloud (Minnesota) Times. Follow her on Twitter at @mfrellick.
For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube.
Source: Read Full Article