Sleep is one of the most essential human activities—so essential, in fact, that if we don’t get enough sleep for even one night, we may struggle to think, react, and otherwise make it through the day. Yet, despite its importance for function and survival, scientists still don’t fully understand how sleep works.
Enter Dragana Rogulja, a neurobiologist on a quest to unravel the basic biology of sleep.
As a self-described latecomer to science, Rogulja found herself drawn to questions she considers “broadly interesting and easy to understand on a basic human level.”
One of these questions…What happens when we sleep?
For Rogulja, an associate professor of neurobiology in the Blavatnik Institute at Harvard Medical School, an intriguing aspect of sleep is the loss of consciousness and awareness it brings, as the outside world disappears and the inner world takes over.
In a conversation with Harvard Medicine News, Rogulja delved into the details of her sleep research, which uses fruit flies and mice to explore why we need to sleep and how we disconnect from the world during sleep.
What are you studying in the context of sleep?
There are two main questions that my lab has been pursuing for the past several years. The first is why sleep is necessary for survival. Why is it that if you don’t sleep, you will literally die after not too long? The other question is how your brain disconnects from the environment when you fall asleep. How are stimuli prevented from reaching your brain during sleep? Elevating the threshold for sensory arousal is essential for sleep, and we want to understand how that barrier is built around the brain. Sleep is one unified state, but it seems to have multiple components that are regulated through separate mechanisms. We want to understand those mechanisms.
How has your research changed how you think about sleep?
For a long time, scientists have been guided by the principle that sleep is of the brain, by the brain, and for the brain. As a result, research has largely focused on the brain in terms of looking for reasons why sleep is necessary for survival. However, we are now realizing that while sleep may be for the brain, it’s not just for the brain. Sleep is a super old behavior that we think originated in the earliest animals. These animals had no brain; they only had a very simple nervous system.
Then, as animals became more complex, these brain-related purposes of sleep evolved. However, researchers have looked at the brains of sleep-deprived animals to try to find a reason why they die, and they haven’t found anything. On the other hand, clinical data show that sleep deprivation in humans leads to all kinds of diseases in the body. To us, this really suggested that sleep is about more than just the brain.
Our research tells us that we need to stop thinking about the brain separately from the body when it comes to sleep. I’m still shocked by the degree to which neuroscientists tend to think about the brain as having superiority over the body and being at the top of a hierarchy. To solve the biggest mysteries in neuroscience, we need to take a more integrated approach, which is what my lab is trying to do for sleep. We have found that we really need to think about the whole body to understand sleep. And it makes sense. When you go to sleep, your muscles relax, your circulation changes. Of course, it’s about the whole body.
What tools do you use to study sleep?
Historically, a lot of sleep research has been done on humans, but those experiments tend to be limited and descriptive, because you can’t really do experimentation on humans. However, over the last two and a half decades, scientists have come to realize that fruit flies sleep; and more recently, we figured out that the genes that regulate sleep in flies are conserved in mice. When I started my lab, we were only using fruit flies as a model system to study sleep, but we have since been able to establish a mouse model as well. Fruit flies allow us to test a lot of hypotheses quickly and do large, unbiased genetic screens, and then we can test what we find out in flies in mice, which, as mammals, are more similar to humans.
In your 2020 Cell paper, you tackled the question of why sleep is necessary for survival. What’s the answer?
We found that fruit flies who slept less had shorter lifespans: We saw a correlation where the more sleep the flies lost, the faster they died. Interestingly, the mode of sleep deprivation did not matter. What mattered was the amount of sleep lost. There seemed to be an inflection point where sleep loss was associated with death, which told us that there might be something specific happening in the body as opposed to general wear and tear.
To investigate this further, we stained different organs in sleep-deprived flies with markers of cell damage. We found that in the gut, there was an increase in oxidizing molecules, and the peak of oxidation correlated with the inflection point where the flies started to die. We confirmed this finding in sleep-deprived mice. But when we gave sleep-deprived flies antioxidants or turned on antioxidant-producing genes in the gut, we found the flies could survive on little or no sleep, suggesting that the gut is a really important target of sleep.
An experimental setup that the Rogulja lab uses to monitor the activity and health of fruit flies. The flies are separated into individual tubes, and their movements are captured by infrared beams. In a 2020 study, the researchers found that the more sleep flies lost, the shorter their lifespans became. Image: Rogulja lab.
Are there any possible applications for humans?
Our findings suggest that if we can prevent oxidation in the gut, we might be able to counteract the effect of losing sleep. This is important because a lot of diseases are tied to gut dysfunction, and many diseases that arise when you don’t sleep enough may actually be a consequence of gut damage. We’re now starting to think about how to diagnose gut oxidation due to lack of sleep in humans. We want to design “swallowables”—pills or tablets you could swallow that report the oxidative state of your gut by, for example, changing the color of your feces.
We’re also looking for biomarkers: molecules already circulating in the body that indicate lack of sleep and gut oxidation. I have physicians in my lab who are profiling sleep-deprived mice to look for such biomarkers. We already have some molecules that are promising markers for oxidation and seem to decrease with antioxidant treatments. Eventually, it may be possible to design supplements that could be taken orally to reverse gut oxidation due to lack of sleep.
You just published a new paper in Cell that explores how the brain disconnects from the environment during sleep. Tell us more.
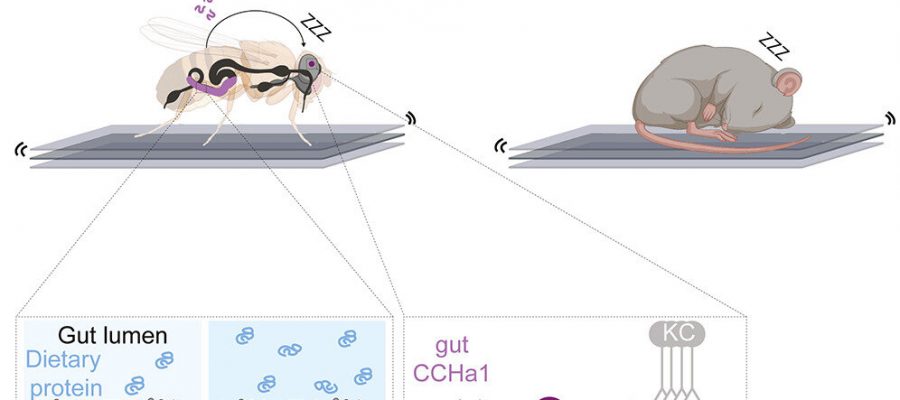
Until now, we knew almost nothing about this. It wasn’t clear if there is a single place in the brain where all sensory information is attenuated during sleep, or if there are multiple such places. For example, are touch and temperature processed the same way during sleep? Iris Titos, a postdoctoral researcher in my lab, built a system that can deliver mild, medium, or high levels of vibration to fruit flies. Typically, when you use low-intensity vibrations, very few flies wake up, and when you use high-intensity vibrations, almost all the flies react. Then, we did a large-scale screen to identify genes that control how easily flies wake up—so genes that make flies super easy to wake up, and genes that allow flies to essentially sleep through an earthquake.
What did the genetic screen show?
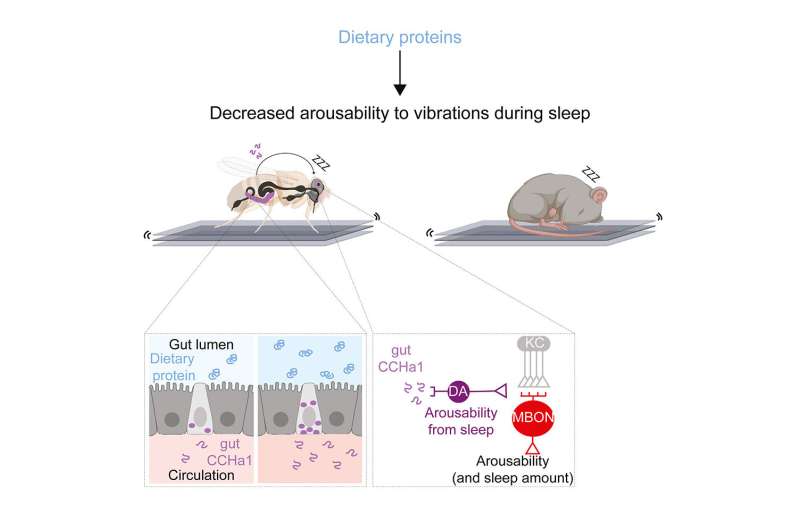
The results of the screen were very interesting. We identified a gene that codes for a molecule called CCHa1. When we depleted CCHa1 in the flies, they woke up very easily—so instead of 20 percent waking up at a particular level of vibration, 90 percent woke up.
However, while CCHa1 is present in both the nervous system and the gut, it was only when we depleted it in the gut that flies were roused more easily. The cells in the gut that produce CCHa1 are called enteroendocrine cells, and they actually share many characteristics with neurons and can even connect and communicate with neurons. These cells face the inside of the gut, and they sort of “taste” the contents of the gut.
We found that the higher concentration of protein in the diet, the more CCHa1 these gut cells produced. This molecule then travels from the gut to the brain, where it signals to a small group of dopaminergic neurons that also receive information about vibrations. These neurons produce dopamine, which usually promotes arousal, but in this case suppresses arousal. Vibrations weaken the activity of the dopaminergic neurons, which causes the flies to wake up more easily. CCHa1 produced by the gut essentially buffers the dopaminergic neurons against vibrations, allowing the flies to ignore the environment to a greater degree and sleep more deeply.
We also found that the CCHa1 pathway, while critical for gating mechanosensory information, has no influence on how easily the flies wake up when exposed to heat, suggesting that different sensory modalities such as vibration and temperature can be gated independently. Finally, we showed that a higher protein diet also improved the quality of sleep in mice, making them more resistant to mechanical disturbances. We are now testing whether a similar signaling pathway is involved in mice.
What do these findings tell you?
Well, we know from other research that when animals are starving, they suppress sleep in order to forage. By contrast, when they’re satiated, and especially when they’re satiated with proteins, they tend to sleep more. Now, we’ve shown that when there’s more protein in the diet, animals also sleep more deeply and become less responsive. This suggests that if animals don’t need to look for food, they can disconnect from the environment and hide somewhere to sleep, which might be safer. More broadly, our study implies that dietary choices impact sleep quality. Now we can explore this connection in humans to understand how diet could be manipulated to improve sleep.
Is there anything about sleep that you think people often misunderstand?
One thing that I think people should be aware of is that how we feel and what’s going on in our bodies don’t have to be the same. In our research, we found that it’s possible to separate the feeling of sleepiness from the need to sleep—some sleep-deprived animals didn’t necessarily feel sleepy, which we could tell because they didn’t sleep extra to catch up on sleep after the deprivation stopped, but these animals still died from the lack of sleep.
This means that even if we can trick ourselves into not feeling sleepy, the lack of sleep still has negative effects on our bodies—for example, if you take a substance that makes you feel awake, the same amount of oxidation is going to happen in your gut. People may say that they’re OK with only a few hours of sleep a night, but they just mean that they can make it through the day. Their bodies are still going to register the lack of sleep. We really cannot tell what’s happening in our bodies as a result of sleep deprivation, and we probably need more sleep than we think we do.
More information:
Iris Titos et al, A gut-secreted peptide suppresses arousability from sleep, Cell (2023). DOI: 10.1016/j.cell.2023.02.022
Journal information:
Cell
Source: Read Full Article