Cherenkov imaging is a valuable cancer treatment tool that can help doctors track and monitor radiation doses received by tissues during cancer therapy in real time. This imaging technique works by detecting Cherenkov radiation, which is emitted by tissues exposed to high-energy radiation, such as X-rays or electron beams from a linear accelerator. As high-energy charged particles from the incident radiation pass through biological tissue, either as primary or secondary radiation, they interact with the electromagnetic fields of the atoms and molecules in the tissue. These soft collision-type interactions lead to electromagnetic shifts in the molecules that release Cherenkov radiation when returning to their original state, which can be detected and correlated to the amount of deposited dose from the radiation.
In an ideal scenario where no Cherenkov is absorbed or scattered by the tissue, the emitted light is directly proportional to the incident radiation dose, allowing for accurate detection of the radiation dose delivered to the tissue from the emitted Cherenkov signal. However in reality, tissue attenuation reduces the intensity of emitted Cherenkov radiation, altering the otherwise linear relationship between the dose deposited and the observed Cherenkov emission. This means that the Cherenkov radiation signal from human tissue is not accurately interpretable as proportional to dose.
Professor Brian W. Pogue currently leads teams at Dartmouth and the University of Wisconsin–Madison that aim to make Cherenkov imaging a reliable indicator of radiation doses. He says, “Previous research on the subject indicates that tissue absorption and scattering could contribute up to 45 percent variation in the detected Cherenkov emission between patients imaged. Further, person-to-person variation in skin color could alter the signal level by up to 90 percent and changes in blood or scattering content can cause up to 20 percent signal variation. This underscores the need to understand Cherenkov signal attenuation in biological tissues through basic studies to see if we can account for this with multispectral or color imaging.”
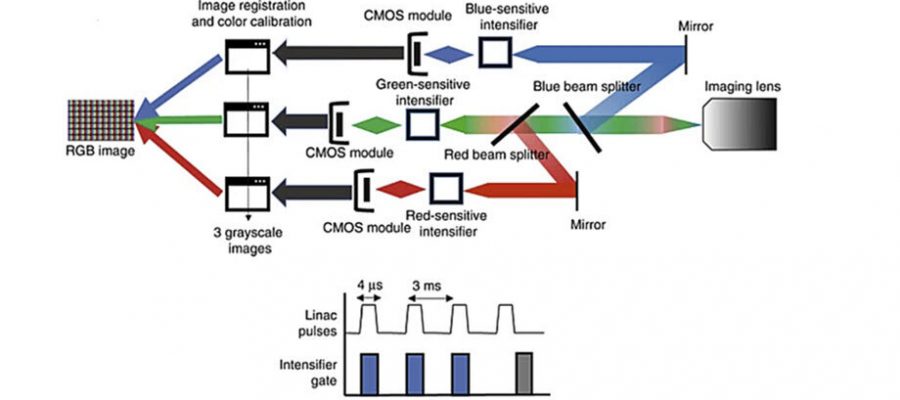
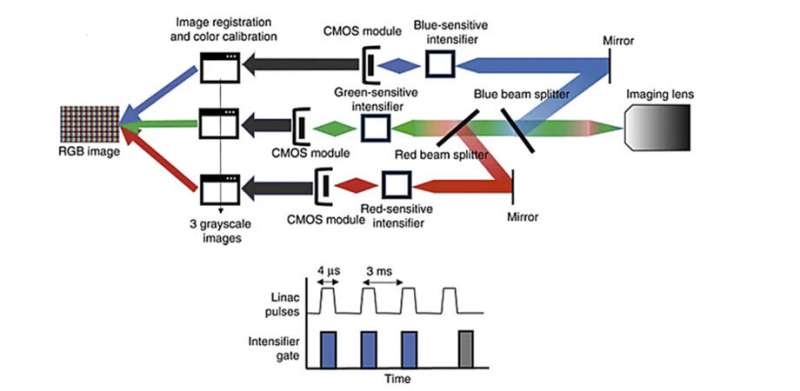
In a study published in Journal of Biomedical Optics (JBO), researchers examined how the intensity of Cherenkov emission changes with variations in biological tissue absorption features such as blood concentration within tissue and melanin concentration in the skin. To do this, they prepared tissue and blood phantoms with varying melanin layers and blood volume levels. They then exposed these phantoms to high-energy X-rays and analyzed the resulting Cherenkov radiation emitted, using a specially designed camera that detected the signal in red, green, and blue (RGB) wavelength bands, as is commonly done in color photography. The key difference is this camera is time-gated to image just during the fast microsecond pulses of radiation.
The researchers used Cherenkov color imaging to determine if they could use information from the spectrum to correct attenuation effects caused by certain biological factors such as blood and melanin levels. They selected melanin concentrations that covered the range of human skin colors and observed that increasing levels of melanin and blood led to a decrease in Cherenkov emission intensity. They noted that extremely high melanin levels can cause a significant reduction in Cherenkov emission, making it challenging to perform Cherenkov color imaging in individuals with the darkest skin tones.
The team found that all colors exhibited a similar reduction in intensity with increasing melanin levels. However, in blood phantoms with increasing blood concentration, they observed the red channel attenuated to a lesser extent than the blue and green channels, due to the absorption of blue and green colors by hemoglobin. They concluded that because the color changes are different, they could calibrate for differences in attenuation based upon either skin color or blood volume in the tissue.
“These results are significant as they demonstrate that distinct biological factors uniquely alter the RGB spectra of Cherenkov emissions. This finding opens the possibility to correct the attenuated Cherenkov signal based on a patient’s blood volume or skin color,” concludes Pogue. “Further development of this work to look at the multispectral signatures is ongoing, as is work on imaging with both the color-regular camera and color-Cherenkov camera.'”
This study demonstrates that Cherenkov color imaging, despite having drawbacks, has the potential to make radiation therapy safer and more effective with appropriate adjustments to the acquired signal.
More information:
Vihan A. Wickramasinghe et al, Color-resolved Cherenkov imaging allows for differential signal detection in blood and melanin content, Journal of Biomedical Optics (2023). DOI: 10.1117/1.JBO.28.3.036005
Journal information:
Journal of Biomedical Optics
Source: Read Full Article