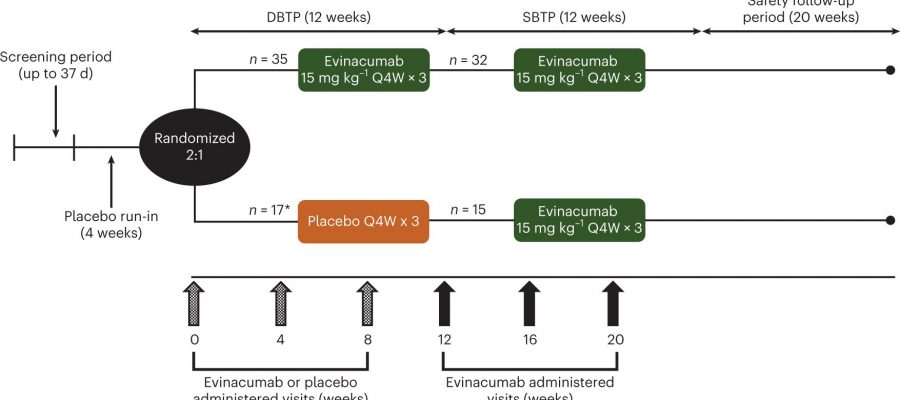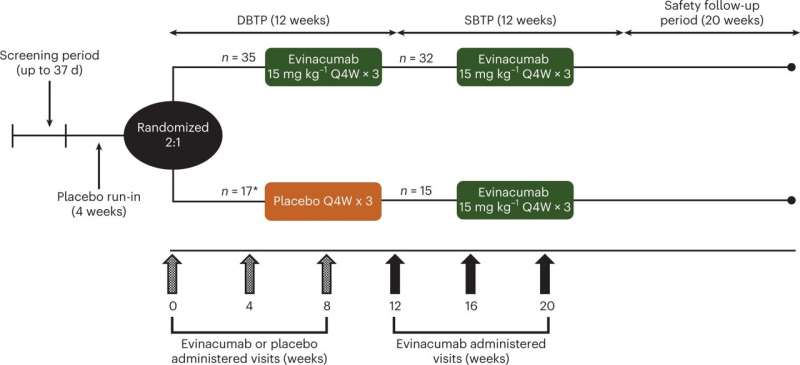
A novel type of therapy, known as ANGPTL3 inhibitor therapy, was effective in lowering triglycerides in certain types of patients with severe hypertriglyceridemia (sHTG) who had a prior episode(s) of acute pancreatitis. sHTG is a well-established risk factor for recurrent episodes of acute pancreatitis. These high-risk patients were the focus of a phase 2 study that was led by the Icahn School of Medicine at Mount Sinai.
This ANGPTL3 inhibitor therapy, the intravenous drug evicanumab-dgnb, inhibits two important regulators of lipoprotein metabolism. The research, published March 6, in Nature Medicine, found that evinacumab reduced triglycerides by as much as 70% in people with sHTG who had at least some lipoprotein lipase (LPL) activity, the key enzyme in triglyceride metabolism.
However, in one of the three groups of sHTG patients with genetic mutations resulting in no LPL activity—a rare condition known as familial chylomicronemia syndrome—evinacumab was ineffective in lowering triglycerides. In the other groups, the triglycerides were reduced by a median of 65% to 82%. Although the primary endpoint of a reduction in triglycerides did not meet the prespecified significance level, many other triglyceride endpoints were positive.
“In many ways we are encouraged by the results of the study,” says lead author Robert S. Rosenson, MD, Director of Lipids and Metabolism for the Mount Sinai Health System, and Professor of Medicine (Cardiology) at Icahn Mount Sinai. “In patients with no residual lipoprotein lipase activity we wouldn’t have expected evinacumab to be effective since there is no enzymatic activity present to disinhibit. But in the broader population of sHTG patients, those with multifactorial chylomicronemia syndrome, it worked profoundly well. Even when the primary endpoints were not met—in familial chylomicronemia syndrome patients—we observed favorable changes in lipid and lipoprotein levels along with safety benefits that warrant further evaluation of this class of inhibitors in larger trials.”
Acute pancreatitis resulting from sHTG impacts 50 to 80 thousand people a year in the United States. It is a significant cause of morbidity, mortality, and reduced quality of life, as well as being a financial burden on the health care system, with patients often experiencing repeat attacks, requiring recurrent hospitalization. Therapeutic approaches to sHTG include weight loss, dietary counseling, lipid l-lowering medicines called fibrates, and omega-3 fatty acids, though none have proven sufficient to reduce triglycerides and prevent acute pancreatitis in a substantial number of patients.
Evinacumab was approved by the U.S. Food and Drug Administration in 2021 as an adjunct to other LDL-lowering therapies for treating patients with homozygous familial hypercholesterolemia, a rare, life-threatening condition that causes severely high cholesterol. Its use in lowering triglycerides was investigational and not approved for use. A fully human monoclonal antibody, it binds to and regulates ANGPTL3, an important regulator of lipoprotein metabolism. It disinhibits key enzymes that break down fats in the body, including lipoprotein lipase, which is crucial to triglyceride metabolism, and endothelial lipase, which is involved in metabolism of low-density lipoprotein (LDL), or “bad,” cholesterol.
“The fact that individuals with loss-of-function variants in ANGPTL3 have significantly reduced triglycerides suggested to us that it could be a good therapeutic target for lowering triglycerides by increasing LPL activity,” explains Dr. Rosenson.
Mount Sinai researchers, in collaboration with colleagues from the Perelman School of Medicine at the University of Pennsylvania, also found that evinacumab treatment in patients with sHTG reduced levels of ApoC3, another important regulator of triglyceride metabolism, by 60%. Patients with sHTG typically have increased ApoC3 levels due to elevation of triglyceride-rich lipoproteins carrying ApoC3. Moreover, the class of ANGPTL3 inhibitors lower levels of apolipoprotein B, a large protein that serves as the backbone of LDL cholesterol and potentially the risk for heart disease.
“We’re particularly encouraged by the reduction we saw in apolipoprotein B, and therefore the potential of this class of inhibitors to prevent cardiovascular events,” says Dr. Rosenson. The effects of evinacumab on cardiovascular morbidity and mortality have not been determined.
“We know that therapies that lower triglycerides without reducing apolipoprotein B do not provide a cardiovascular benefit,” emphasizes Dr. Rosenson. “By significantly impacting both risk factors, ANGPTL3 inhibitors show great therapeutic promise for large populations of patients, which our seminal work is now helping to accelerate for the broad field of clinical research.”
In the study, treatment-emergent adverse events occurred in 71% and 69% of evinacumab and placebo-treated patients, respectively (a secondary endpoint). Common treatment emergent adverse events occurring in more than 5 percent of patients that were more frequent in the evinacumab group included abdominal pain, headache, constipation, abdominal discomfort, increase in alanine aminotransferase/aspartate aminotransferase (defined as three times the upper limit of normal), back pain, contusion, dizziness, herpes zoster, and sinusitis.
More information:
Robert S. Rosenson et al, Evinacumab in severe hypertriglyceridemia with or without lipoprotein lipase pathway mutations: a phase 2 randomized trial, Nature Medicine (2023). DOI: 10.1038/s41591-023-02222-w
Journal information:
Nature Medicine
Source: Read Full Article