In a recent preprint study published on the medRxiv* preprint server, researchers determine the serum neutralizing and binding activity against the severe acute respiratory syndrome-associated coronavirus 2 (SARS-CoV-2) Omicron variant in individuals with different levels of immunity against coronavirus disease (COVID-19).
Study: Activity of convalescent and vaccine serum against a B.1.1.529 variant SARS-CoV-2 isolate. Image Credit: Fit Ztudio / Shutterstock.com
Emergence of the Omicron variant
Soon after the first case of the SARS-CoV-2 Omicron variant (B.1.1.529) was reported in South Africa and Botswana in November 2021, the Omicron variant quickly spread to many countries around the world.
The Omicron variant is considered to be more infectious than the Delta variant due to a large number of mutations in the spike (S) protein region and around 15 amino acid changes in the receptor-binding domain (RBD). Previous studies indicate that the highly antigenically distinct SARS-CoV-2 B.1.1.529 variant is associated with decreased vaccine effectiveness (VE) and extensive immune evasion.
About the study
The present study determined the serum neutralizing and binding activity against SARS-CoV-2 wild-type, Beta (B.1.351), and Omicron strains in convalescent COVID-19, two-dose messenger ribonucleic acid (mRNA) vaccinated, mRNA booster vaccinated, and convalescent two-dose, and booster-vaccinated participants. The study participants received either Pfizer or Moderna vaccines.
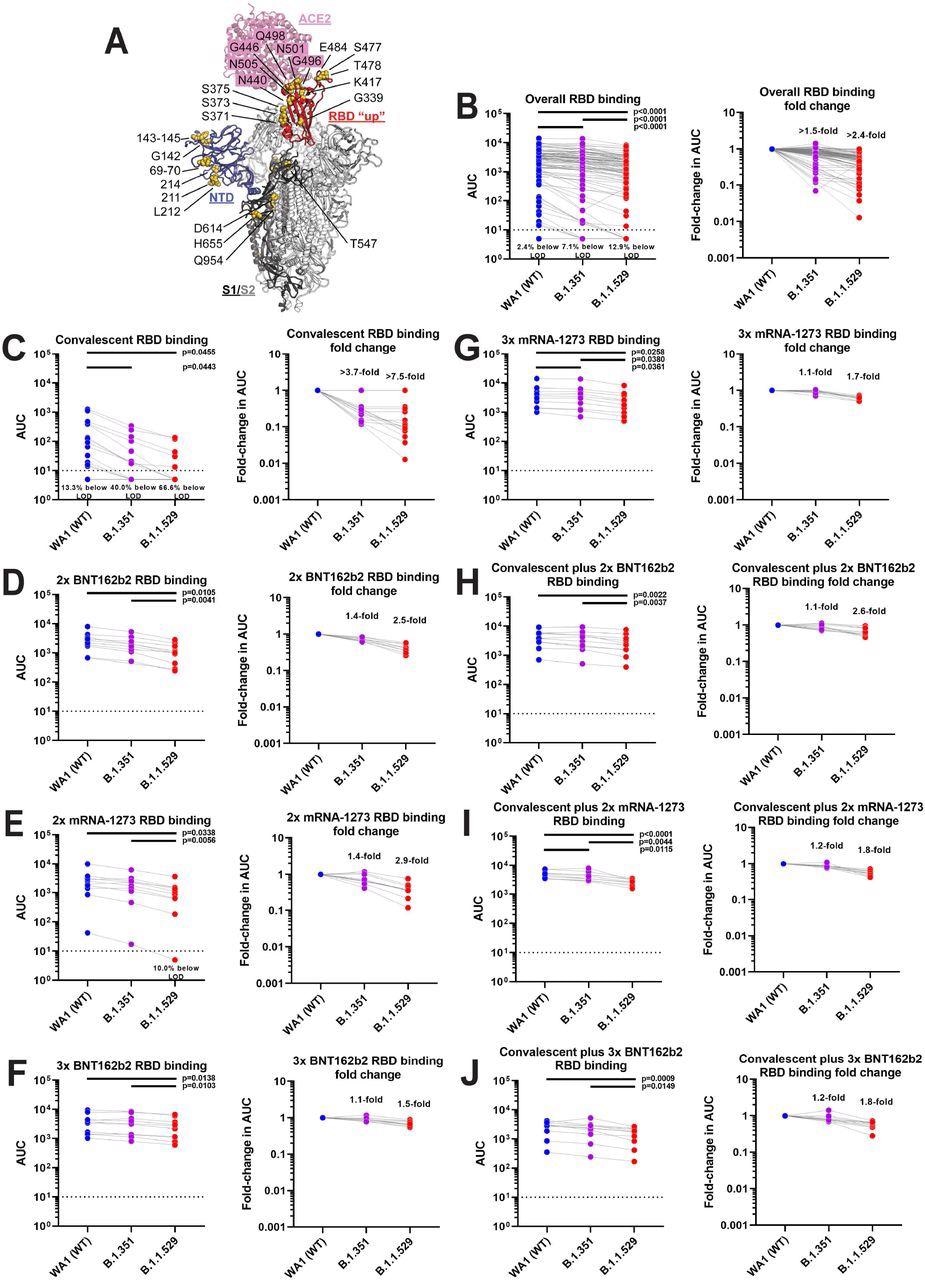
Sera of vaccinated individuals mostly maintain binding to the B.1.1.529 RBD. A shows a model of the B.1.1.529 spike protein in complex with the angiotensin-converting enzyme 2 (ACE2) receptor with B.1.1.529 specific mutations indicated. The figure is based on PDB 6M0J and 7C2L and was built in PyMol. B shows absolute titers (left) and fold reduction (right) for the combined samples, C to J shows the different groups. A one-way ANOVA with Tukey’s multiple comparisons test was used to compare the neutralization titers and significant p values (<0.05) are indicated in the figure. The exception is panel D were a mixed-effects model had to be used due to a missing data point.
Convalescent and post-vaccination sera were collected from the subjects of the longitudinal observational Protection Associated with Rapid Immunity to SARS- CoV-2 (PARIS) study after receiving written informed consent. The Omicron variant was antigenically characterized using the 85 serum samples collected from 54 participants.
The SARS-CoV-2 biospecimens collected from the Mount Sinai Pathogen Surveillance program were sequenced either based on spike S1 mutational profile or using an established complete virus genome sequencing approach.
Recombinant RBD proteins were produced and cloned into the mammalian expression vector pCAGGS using Expi293F cells. These proteins were then purified after transient transfections with each respective plasmid. Similarly, the nucleocapsid (N) terminal domain (NTD) protein was cloned into the pVRC8400 expression vector and was transiently expressed in FreeStyle™ 293-F cells.
The antibody titers in serum from convalescent COVID-19 patients and vaccine recipients were analyzed by enzyme-linked immunosorbent assay (ELISA) using recombinant RBD and NTD of SAR-CoV-2 wild-type (WA1), Beta, and Omicron strains. A multicycle microneutralization assay was also performed using vaccine recipients’ serum to determine the antibody neutralization ability of SARS-CoV-2 wild-type, Beta, and Omicron isolates.
Study findings
In the 85 samples studied, there was a more than a 14.5-fold reduction in neutralization against the Omicron variant. Further, 16.5% of samples showed no neutralization against the Omicron variant. Meanwhile, there was only a four-fold reduction in neutralizing capacity against the Beta variant.
Convalescent COVID-19 patients showed low antibody neutralization against SARS-CoV-2 wild-type and Beta variants and no neutralizing activity against the Omicron variant. Most of the individuals who received a two-dose Moderna or Pfizer vaccination regimen showed low but detectable antibody neutralization and had a 42-fold and 23-fold reduction, respectively, in neutralization activity against the Omicron variant. Comparatively, Moderna and Pfizer booster vaccine recipients had a lower reduction in neutralization with a 16.7-fold and 7.5-fold drop, respectively.
Convalescent COVID-19 patients who received two doses of Moderna or Pfizer vaccines, as well as those who received three doses of the Pfizer vaccine showed 11-fold, 14-fold, and 13-fold decrease, respectively, in Omicron neutralization. Robust antibody neutralization was maintained in these three cohorts.
A 7.5-fold reduction in antibody binding to the RBD in Omicron was observed in two-thirds of convalescent COVID-19 patients, who showed no detectable activity following ELISA. However, in other groups, the RBD binding was well maintained. Similarly, the antibody binding to NTD was also well maintained with some minor reductions.
Conclusions
The researchers conclude that there is a significant decline in serum neutralizing activity against the Omicron variant in convalescent COVID-19 patients and two-dose vaccinated participants. Although at decreased levels, the serum neutralizing activity in two-dose or booster vaccinated convalescent COVID-19 patients were maintained. The Omicron RBD and NTD binding capacity declined in unvaccinated convalescent COVID-19 patients, whereas it was mostly retained in vaccinated convalescent patients.
The findings of the current study align with previous reports on the impact of Omicron on the neutralization activity of convalescent COVID-19 patients’ and vaccine recipients’ serum. This suggests that those who received booster doses in convalescent vaccinated groups have significant protection against the Omicron variant.
According to the authors, this is the first study that details RBD- and NTD-specific binding changes with respect to the Omicron variant and adds on evidence pointing to the need for Omicron-specific vaccines.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Carreño, J. M., Alshammary, H., Tcheou, J., et al. (2021). Activity of convalescent and vaccine serum against a B.1.1.529 variant SARS-CoV-2 isolate. medRxiv. doi:10.1101/2021.12.20.21268134. https://www.medrxiv.org/content/10.1101/2021.12.20.21268134v1
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: Amino Acid, Antibody, Assay, Coronavirus, Coronavirus Disease COVID-19, Enzyme, Genome, immunity, Pathogen, Plasmid, Protein, Receptor, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine, Virus

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article
